The People’s Court: Case No. 25-CV-1027
OPENING STATEMENTS
PROSECUTION (Representing: Health Insurance Companies & Big Pharma)
“Your Honor, we’re not here to dispute that GLP-1 receptor agonists work. We fully support their use for diabetes and obesity at FDA-approved doses. We stipulate to that.
But what we’re seeing now is medical vigilantism. Practitioners prescribing semaglutide 0.15 mg, 0.25 mg per week – “Microdoses” -for conditions never studied, at doses never proven effective, based on a daisy chain of:
- Mouse studies that don’t translate
- Dose-response curves that show threshold effects requiring higher doses
- Social media testimonials
- And wishful thinking
From an insurance perspective, we cannot reimburse treatments without evidence of efficacy. Our responsibility is to our members and shareholders. We need proof that we’re paying for interventions that actually work.
From a pharmaceutical perspective, our approved dosing regimens exist for a reason. We spent billions proving that 2.4 mg of semaglutide works for obesity. If lower doses were effective, we would have studied them. The fact that we didn’t should tell you something.
The defense will show you impressive preclinical data. Yes, GLP-1RAs like semaglutide and tirzepatide have anti-inflammatory effects in test tubes and rodents. But translation to humans at subtherapeutic doses? Nonexistent.
We have evidence. Real evidence:
- A 12-week study: submaximal semaglutide improved metabolic markers but showed no change in the inflammatory markers IL-6, TNF-α, or PAI-1
- Semaglutide dose-response studies: effects require ≥0.2 mg/day, and even those effects are diminished when adjusted for weight change
- Meta-analyses showing anti-inflammatory effects correlate with improvements in insulin resistance and weight loss, not with microdosing voodoo magic.
The human user is being sold a fantasy: all the benefits, none of the side effects, at doses that haven’t been proven to do anything.
This isn’t medicine. It’s marketing masquerading as innovation. And we’re not paying for it.”
DEFENSE (Representing: Autoimmune Disease, PCOS, Addiction, Osteoarthritis, Cancer, Cardiovascular Disease, Overweight-Without-Approval and “Longevity”):
“Your Honor, the prosecution wants you to believe that unless something is proven in a Phase III randomized controlled trial, it doesn’t exist. And conveniently, they’re the ones who decide which trials get funded.
Let’s be clear about the conflicts here: Big Pharma profits from high-dose, on-label prescriptions. Insurance companies save money by denying coverage for anything off-label. Neither has an incentive to prove that low-dose generic semaglutide works, even if it does.
Let’s talk about what we actually know:
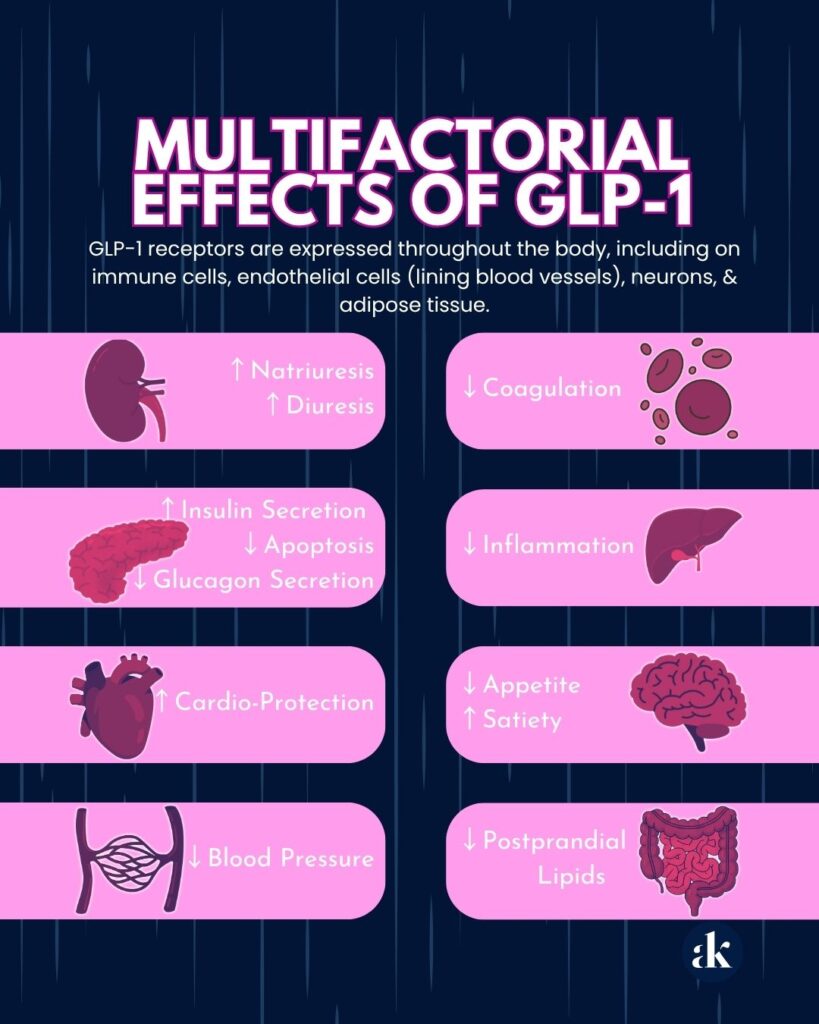
The Biology: GLP-1 receptors are expressed throughout the body, including on immune cells, endothelial cells (lining blood vessels), neurons, and adipose tissue. This isn’t speculative; it’s basic receptor biology. See Exhibit 1 below.
The Dose-Response Question: Yes, higher doses produce more dramatic effects. That’s not controversial. We don’t have studies specifically designed to find the minimal effective dose for anti-inflammatory effects because no one has funded them. The studies we have asked: ‘Does 2.4 mg work better than 1.0 mg?’ Not: ‘What’s the lowest dose that provides benefit?’
The Weight Loss Confound: The prosecution says effects disappear when you control for weight loss. But we need to unpack what ‘weight loss’ actually means, because they’re conflating several distinct phenomena:
First, you don’t need to be overweight to benefit from fat loss. A normal-weight person with excess visceral fat, the inflammatory, metabolically active fat surrounding organs, can experience dramatic metabolic improvements from losing just that visceral depot. The scale might barely move, but insulin sensitivity, inflammatory markers, and liver function can transform.
Second, and this is critical: we’re seeing consistent reports of benefits WITHOUT any weight loss at all. Normal-weight individuals reporting reduced joint pain, improved autoimmune symptoms, decreased brain fog, and normalized menstrual cycles with zero change on the scale. Are these all the placebo effect? Are they all lying? Or is there something happening beyond simple caloric restriction?
Clinical Reality: We have hundreds of clinicians observing:
- PCOS patients with cycle normalization at semaglutide 0.25 mg/week, some with weight loss, some without.
- Normal-weight autoimmune patients reporting decreased joint pain and skin rashes without losing a pound. Entire Reddit channels dedicated to talking about GLP-1s as “Autoimmune disease miracles” noting improvements in symptoms on a variety of GLP-1 doses.
- Addiction patients with reduced cravings whose weight remains stable.
- People who are unable to tolerate higher doses but still experience meaningful benefits when experimenting with lower “off label” doses.
Our Position: Should microdosing be studied rigorously? Yes. Should we pretend that mechanistic plausibility plus consistent clinical observation equals zero evidence? No.
We’re asking for the same latitude given to off-label prescribing in every other domain of medicine: informed consent, careful monitoring, intellectual honesty about uncertainty.”
EXPERT WITNESS TESTIMONY
DEFENSE calls Dr. Metabolism to the stand:
Defense: “Doctor, the prosecution keeps saying ‘the benefits are just from weight loss.’ Can you clarify what that actually means?”
Dr. Metabolism: “It’s reductive, frankly. When we say ‘weight loss,’ we’re usually talking about several different processes:
- Loss of subcutaneous fat – the fat you can pinch
- Loss of visceral fat – the inflammatory fat around organs
- Changes in adipocyte function – how fat cells behave
- Systemic metabolic reprogramming
These don’t always happen together, and they don’t always correlate with the number on the scale.”
Defense: “So someone could have metabolic improvement without significant weight loss?
Dr. Metabolism: “Absolutely. A person with normal BMI but high visceral fat could lose 3 pounds of mostly visceral fat and see dramatic improvements in insulin sensitivity, liver enzymes, and inflammatory markers. Their weight barely changed, but their metabolic health transformed.”
Defense: “And we’re seeing reports of benefits in people with no weight loss at all. How do you explain that?”
Dr. Metabolism: “Several possibilities. First, GLP-1 receptors on immune cells may directly modulate inflammatory responses independent of any fat loss. Second, GLP-1 affects the gut microbiome, intestinal permeability, and gut-derived inflammation, none of which require weight loss. Third, the central nervous system’s effects on reward processing, stress response, and autonomic balance could produce benefits without altering body composition. Fourth, improved glycemic variability and reduced postprandial (post-meal) glucose excursions can reduce oxidative stress even in normal-weight individuals without diabetes.”
Defense: “So the ‘it’s just weight loss’ argument…”
Dr. Metabolism: “Is an oversimplification that ignores the complexity of GLP-1 biology.”
PROSECUTION cross-examines:
Prosecutor: “Doctor, these reports of benefits without weight loss – how many are published in peer-reviewed journals?”
Dr. Metabolism: “Very few, but -”
Prosecutor: “Very few. So we’re talking about anecdotes, social media posts, unpublished observations. Correct?”
Dr. Metabolism: “Clinical observations, yes.”
Prosecutor: “And placebo effects in pain conditions, autoimmune symptoms, and subjective measures like ‘brain fog’ – those can be quite strong, can’t they?”
Dr. Metabolism: “Yes, but – “
Prosecutor: “In fact, placebo response rates in pain trials can be 30-40%, correct?”
Dr. Metabolism: “That’s true.”
Prosecutor: “So when someone on a trendy medication says their joint pain improved, and their weight didn’t change, the most likely explanation is placebo effect, isn’t it?”
Dr. Metabolism: “Unless we’re seeing consistent patterns across thousands of patients.”
Prosecutor: “Consistent patterns that aren’t being objectively measured or published. Doctor, you mentioned visceral fat loss in normal-weight individuals. How much visceral fat loss is required to produce anti-inflammatory benefits?”
Dr. Metabolism: “It varies by individual, but -”
Prosecutor: “And at microdoses, how much visceral fat loss do we see?”
Dr. Metabolism: “…We don’t have good data on that specific question.”
Prosecutor: “We don’t have data. So you’re speculating that microdoses produce just enough visceral fat loss, perhaps undetectable on a scale, to explain benefits. Is that fair?”
Dr. Metabolism: “I’m saying it’s a possible mechanism, yes.”
Prosecutor: “Possible. But unproven. Along with all your other possible mechanisms: gut microbiome, direct immune effects, CNS effects. All possible. All unproven at microdoses. Thank you, Doctor.”
CLOSING ARGUMENTS
PROSECUTION:
“Your Honor, the defense has constructed an elegant narrative:
- Maybe it’s visceral fat loss that doesn’t show on the scale
- Maybe it’s direct anti-inflammatory effects
- Maybe it’s gut microbiome changes
- Maybe it’s CNS effects
But Your Honor, ‘maybe’ is not a medical standard.
Yes, a normal-weight person could benefit from visceral fat loss. But do we know microdoses produce that? No.
Yes, there could be anti-inflammatory effects independent of weight loss. But do we have human data at microdoses? No.
We have a healthcare system to protect. If we start covering every off-label use based on Reddit testimonials and mechanistic hand-waving, costs become unsustainable, and we incentivize medical anything-goes behavior.
This is science fiction masquerading as clinical practice. I’m surprised we don’t see dragons flying in the skies with all the fairy-dusting you’re spreading around.
Evidence first. Then clinical practice. Not the reverse.”
DEFENSE:
“Your Honor, let me address the prosecution’s core demand: prove it first, then use it.
That’s not how medicine works. That’s not how it’s ever worked.
When a clinician sees a pattern of disease improvement using a drug off-label, they don’t say ‘Interesting, but I’ll wait 10 years for the RCT.’ They say: ‘This patient is suffering. I have a mechanistically plausible intervention with minimal risk. Let’s try it.’
And let’s talk about the insurance companies’ argument about protecting the healthcare system. If they were truly concerned about long-term costs, they’d be funding these microdosing studies, not blocking coverage.
The prosecution is protecting quarterly earnings, not long-term fiscal health. They’re penny-wise and pound-foolish, saving money today by denying a $50/month compounded medication, then paying $50,000 for the bypass surgery in five years.
The prosecution wants to wait for perfect evidence. But patients are:
- Suffering from autoimmune disease NOW
- Struggling with PCOS NOW
- Fighting addiction NOW
- Accumulating visceral fat in midlife NOW
- Developing cardiovascular disease and cognitive decline NOW
We’re offering them:
- A biologically plausible intervention
- With an excellent safety profile at low doses
- Under careful monitoring
- With full disclosure of evidence limitations
The prosecution is offering them:
- A decade of continued suffering
- While we wait for studies that pharmaceutical companies won’t fund
- Because there’s no profit in proving generic semaglutide works at 0.15 or 0.25 mg for off-label indications.
Should we investigate further? Absolutely. Should we withhold potentially beneficial treatment while we wait? That’s not medicine – that’s bureaucracy.
THE VERDICT
All rise. The honorable Amy Killen, MD, will now address the court with her findings in this case.
“I’ve listened to both sides, reviewed the evidence, and I’m struck by how this case illuminates the complexity of metabolic health.
Here’s what I conclude:
GLP-1 microdosing and off-label use occupy an uncomfortable gray zone – strong mechanistic rationale, consistent observational patterns, but limited controlled human data.
If you’re a clinician considering microdosing:
- Be explicit: “We don’t know exactly how or why this might work at this dose”
- Consider it especially for:
- Normal-weight patients with metabolic dysfunction
- Patients who can’t tolerate higher doses
- Conditions with strong mechanistic rationale (PCOS, autoimmune disease, addiction)
- Monitor objectively: weight and body composition, waist circumference, labs, symptom scores, not just subjective reports
- Track patients who don’t lose weight but report benefits – these are your most interesting cases
- Document everything, contribute to registries
If you’re a patient considering microdosing:
- Understand that benefits without weight loss are reported but not proven
- Know that you might be benefiting from small amounts of visceral fat loss that don’t show on your scale
- Be skeptical of your own subjective improvement (placebo is real)
- Know that all medications come with possible side effects
- Work with a clinician who will help you figure out if it’s actually working
If you’re a researcher:
- Please study the minimal effective dose
- Use imaging to measure visceral fat, not just scale weight
- Design studies in normal-weight metabolically unhealthy individuals
- Compare weight-matched groups to isolate non-weight-loss effects

My opinion?
The “it’s all just weight loss” argument is already showing cracks in its armor. The body is more complex than that.
Most likely reality: microdoses produce small metabolic improvements – subtle visceral fat changes, modest improvements in insulin sensitivity, minor reductions in inflammatory signaling – that compound over time into meaningful clinical benefits. Some of these happen independent of any weight loss. Some happen because of weight loss you can’t see on a scale or because of modifications to food intake.
Proceed thoughtfully. Document carefully. Be humble about uncertainty. And stop pretending that absence of published data equals absence of effect.
As to the fairy-dusting dragons in the sky – tell Tairn and Andarna Hi for me. 🙂
Court adjourned.“
References:
Bendotti G, Montefusco L, Lunati ME, et al.
Pharmacological Research. 2022;182:106320. doi:10.1016/j.phrs.2022.106320.
Karacabeyli D, Lacaille D.
Nature Reviews. Rheumatology. 2025;:10.1038/s41584-025-01302-0. doi:10.1038/s41584-025-01302-0.
Bray JJH, Foster-Davies H, Salem A, et al.
Diabetes, Obesity & Metabolism. 2021;23(8):1806-1822. doi:10.1111/dom.14399.
Newsome P, Francque S, Harrison S, et al.
Alimentary Pharmacology & Therapeutics. 2019;50(2):193-203. doi:10.1111/apt.15316.
Knop FK, Aroda VR, do Vale RD, et al.
Lancet (London, England). 2023;402(10403):705-719. doi:10.1016/S0140-6736(23)01185-6.
Péč MJ, Jurica J, Péčová M, et al.
Pharmaceuticals (Basel, Switzerland). 2025;18(9):1364. doi:10.3390/ph18091364.
Reppo I, Jakobson M, Volke V.
International Journal of Molecular Sciences. 2023;24(6):5714. doi:10.3390/ijms24065714.
Gonzalez-Rellan MJ, Drucker DJ.
JAMA. 2025;:2838996. doi:10.1001/jama.2025.14392.
O’Keefe JH, Franco WG, O’Keefe EL.
Progress in Cardiovascular Diseases. 2025 Mar-Apr;89:102-112. doi:10.1016/j.pcad.2024.12.010.
Badulescu S, Tabassum A, Le GH, et al.
Physiology & Behavior. 2024;283:114622. doi:10.1016/j.physbeh.2024.114622.
Ryan M, Megyeri S, Nuffer W, Trujillo JM.
Pharmacotherapy. 2025;45(3):177-186. doi:10.1002/phar.70005.
Bilgin E, Venerito V, Bogdanos DP.
Autoimmunity Reviews. 2025;24(9):103864. doi:10.1016/j.autrev.2025.103864.
Karagiannis T, Malandris K, Avgerinos I, et al.
Diabetologia. 2024;67(7):1206-1222. doi:10.1007/s00125-024-06144-1.
Elmaleh-Sachs A, Schwartz JL, Bramante CT, et al.
JAMA. 2023;330(20):2000-2015. doi:10.1001/jama.2023.19897.
Singh A, Singh AK, Singh R, Misra A.
Diabetes & Metabolic Syndrome. 2025;19(3):103212. doi:10.1016/j.dsx.2025.103212.
- Role of glucagon-like peptide 1 agonists in obesity and heart failure with preserved ejection fraction.
Temporelli, Pier Luigi.
European Heart Journal Supplements. 26(Supplement_1):i127-i130. DOI:10.1093/eurheartjsupp/suae011













